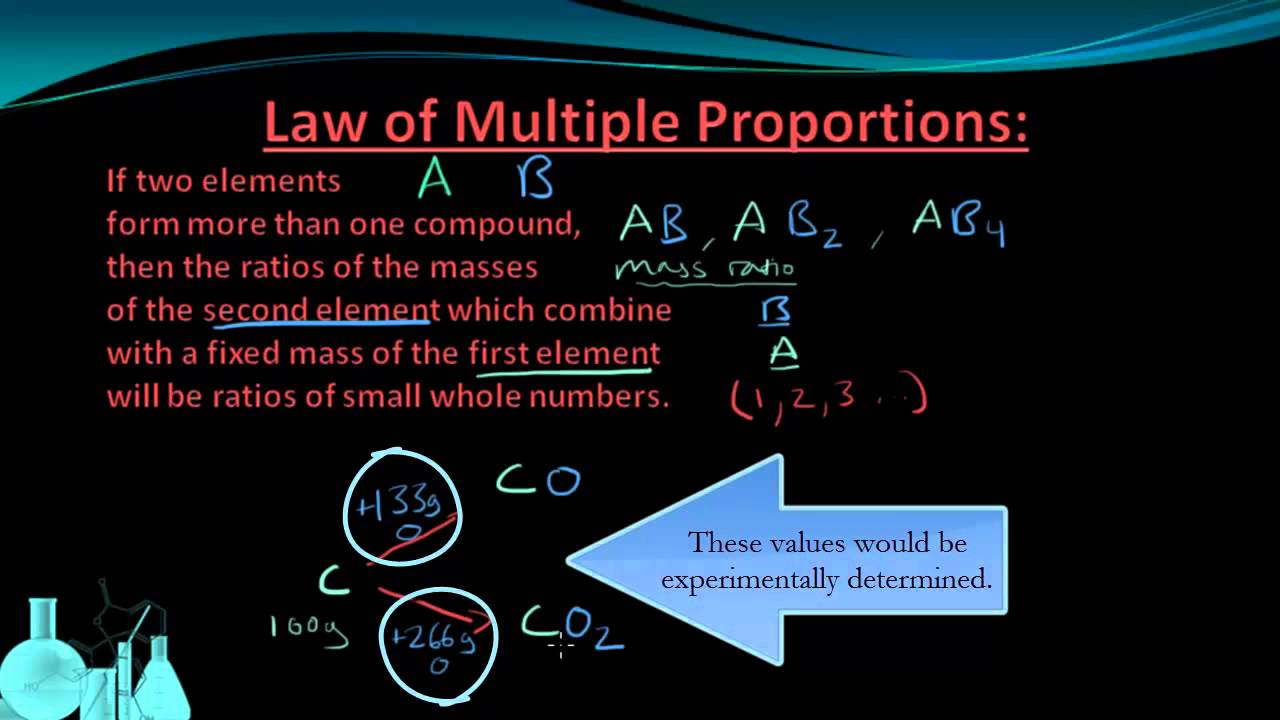
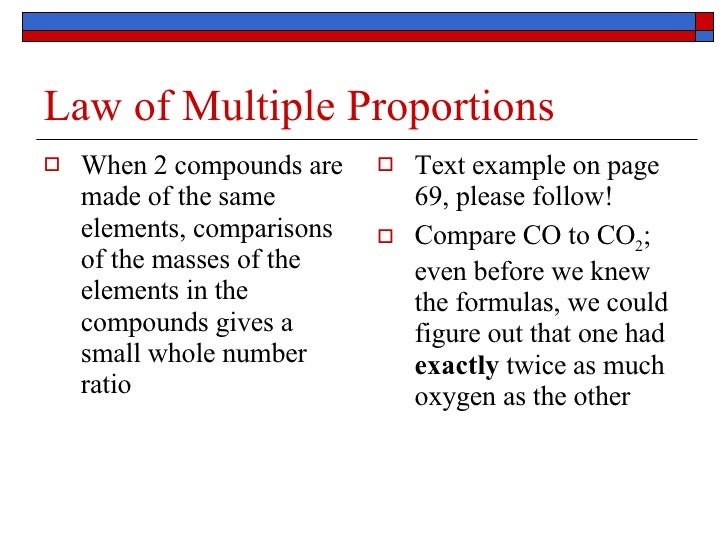
Law of Multiple Proportions

We have a lot of food options because we love variety, and we like to try new things. Sometimes, we like to try different combinations of our favorite things. Let's examine the toppings on this pizza: it has tomatoes, pepperoni, bell pepper, cheese, olives and mushrooms. Sometimes, we like to double the cheese and double the pepperoni. There are other times when we don't feel like having a lot of mushrooms, so we just put it on half the pizza.
Now, let's think about a nice, classic breakfast item, which is the ham, egg and cheese sandwich. Sometimes we double up on the egg, or we double up the cheese or the ham when we feel hungry. Sometimes we just eat half the sandwich if we are in a hurry. There are many possible combinations when it comes to food.
Just like different combinations are possible in food, different combinations are also possible for elements that make up compounds. Compounds are made up of atoms of different elements. There are compounds made up of the same elements, like carbon monoxide (CO) and carbon dioxide (CO2). Both compounds are made of carbon (C) atoms and oxygen (O) atoms; however, the ratios of carbon and oxygen in each compound is different. This illustrates the law of multiple proportions.
The law of multiple proportions, states that when two elements combine to form more than one compound, the mass of one element, which combines with a fixed mass of the other element, will always be ratios of whole numbers.
Let us remember that the law of multiple proportions only applies to compounds composed of the same elements. It does not apply to, let's say, SO2 and CO2 because one compound has sulfur (S) and one has carbon (C).
Demonstrating the Law of Multiple Proportions
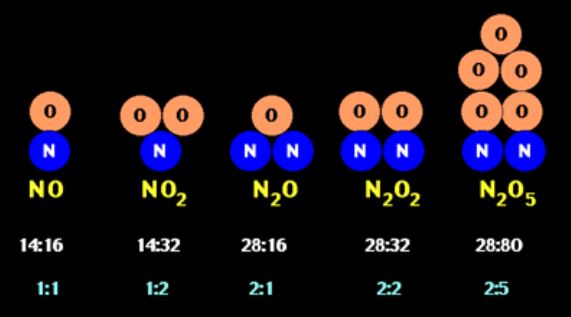
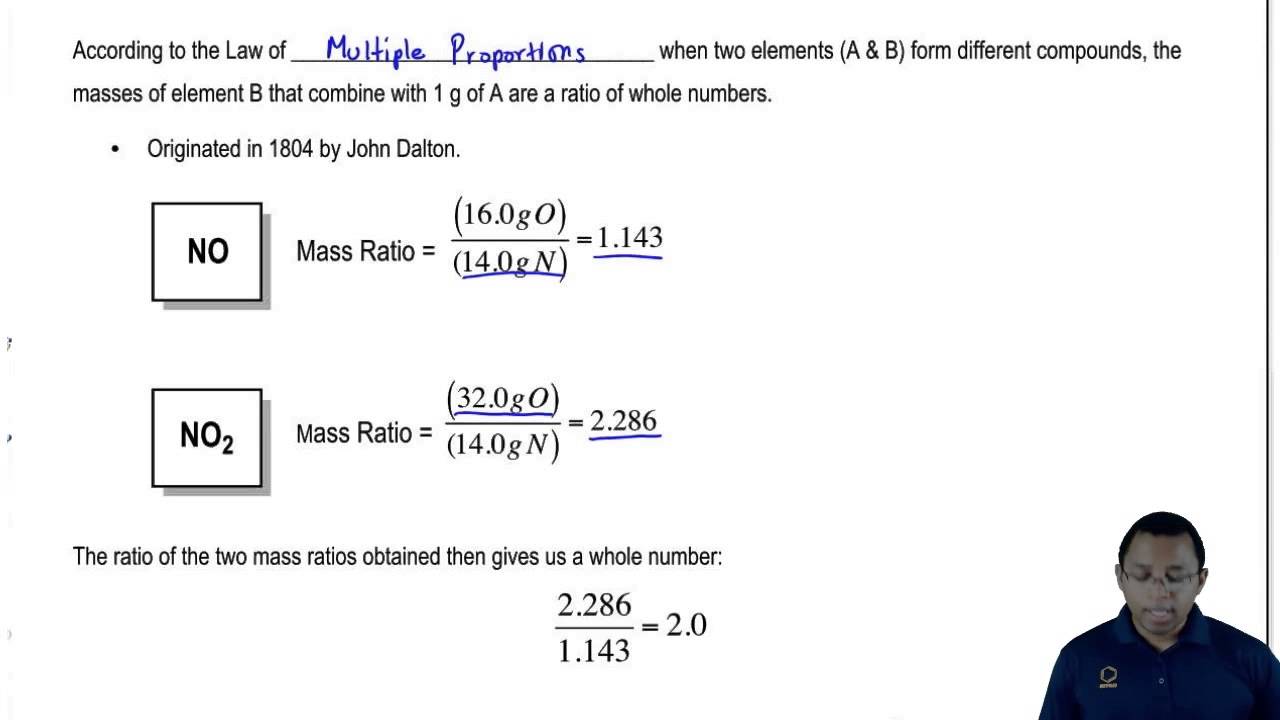
Let us demonstrate the law of multiple proportions with these two elements: nitrogen monoxide and nitrogen dioxide.
Nitrogen monoxide is made of one nitrogen (N) atom and one oxygen (O) atom, forming (NO), and nitrogen dioxide is made of one nitrogen (N) atom and two oxygen (O) atoms, forming (NO2). Here is how the elements nitrogen and oxygen appear on the periodic table, and encircled in red are their atomic masses:
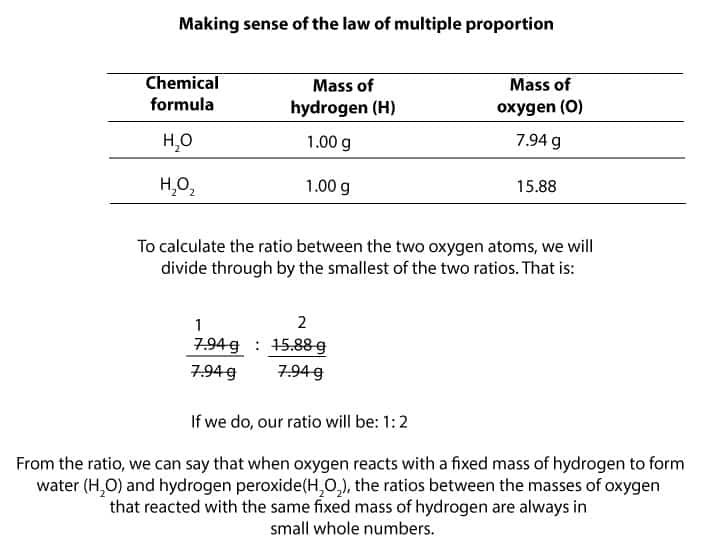
If we round the atomic masses, nitrogen has an atomic mass of 14 and oxygen has an atomic mass of 16. So, for nitrogen monoxide, we can say that it is made of 14 parts by mass nitrogen and 16 parts by mass oxygen - the ratio of nitrogen to oxygen is therefore 14:16. For nitrogen dioxide, we can say it is made of 14 parts by mass nitrogen and 32 parts by mass oxygen - the ratio of nitrogen to oxygen is, therefore, 14:32. Let us summarize this information in this table:
The next thing we do is we divide both ratios by the smallest number, in this case, 14.
The ratios mean that for nitrogen monoxide (NO), for every 1 g of nitrogen, there is 1.143 g of oxygen. For nitrogen dioxide (NO2), for every 1 g of nitrogen, there are 2.286 g of oxygen.
How does the law of multiple proportions apply to this? We can demonstrate it by taking the ratios of the oxygen for the two compounds and putting them side by side. Then we divide it by the smaller number, which is 1.143:
Gallery Law Of Multiple Proportions
 Law Of Multiple Proportions By Alessandra Pb On Prezi
Law Of Multiple Proportions By Alessandra Pb On Prezi
Chem 103 Lab The Law Of Multiple Proportions
 Solution Which Of The Following Sets Ill Chemistry
Solution Which Of The Following Sets Ill Chemistry
 Chemistry 11 Law Of Definite Composition Multiple
Chemistry 11 Law Of Definite Composition Multiple
 Law Of Definite Proportions Law Of Multiple Proportions Concept
Law Of Definite Proportions Law Of Multiple Proportions Concept
Practice Law Of Definite And Multiple Proportions Workshe
 Basic Laws Of Chemistry Law Of Multiple Proportions
Basic Laws Of Chemistry Law Of Multiple Proportions
 Chemistry 5 8b Law Of Multiple Proportions
Chemistry 5 8b Law Of Multiple Proportions
Law Of Multiple Proportions Home
 Law Of Multiple Proportions Definition Examples Video
Law Of Multiple Proportions Definition Examples Video
 How The Law Of Constant Composition Conservation Of Mass
How The Law Of Constant Composition Conservation Of Mass
 Law Of Constant Proportions Explanation And Exceptions
Law Of Constant Proportions Explanation And Exceptions
 Law Of Definite Proportions Law Of Multiple Proportions
Law Of Definite Proportions Law Of Multiple Proportions
 Law Of Multiple Proportions And Law Of Definite Proportions
Law Of Multiple Proportions And Law Of Definite Proportions

 Law Of Definite Composition And Law Of Multiple Proportions
Law Of Definite Composition And Law Of Multiple Proportions
 File Demonstration Of The Law Of Multiple Proportions Jpg
File Demonstration Of The Law Of Multiple Proportions Jpg
 Videos Matching Law Of Multiple Proportion Revolvy
Videos Matching Law Of Multiple Proportion Revolvy

 Law Of Multiple Proportions And Law Of Definite Proportions
Law Of Multiple Proportions And Law Of Definite Proportions
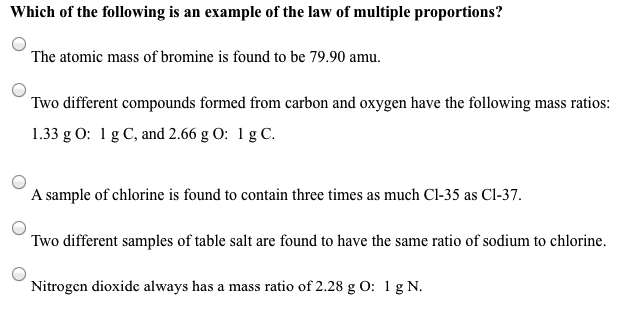 Solved Which Of The Following Is An Example Of The Law Of
Solved Which Of The Following Is An Example Of The Law Of
Comments
Post a Comment