 The Law Of Definite Composition Introduction To Chemistry
The Law Of Definite Composition Introduction To Chemistry
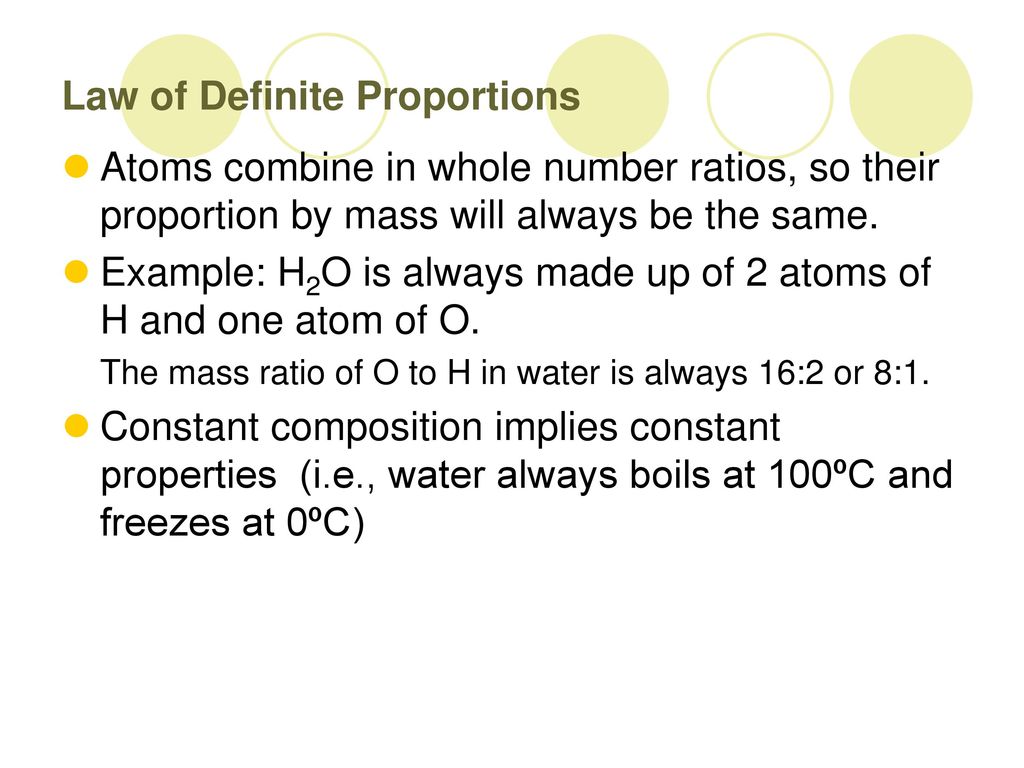
The Law of Definite Proportions

Have you ever wanted to make more food than a recipe called for? Let's say you have a chocolate cake recipe that feeds four people, but you're going to a birthday party that will have eight guests. You will need to double the recipe, which also means you'll need to double the amount of eggs, sugar, flour, butter, milk and chocolate for the recipe. The amount of each ingredient will double, but the proportions should stay the same. We can think about this principle when defining The Law of Definite Proportions, which states that any chemical compound will always contain a fixed ratio of elements by mass.
A French scientist named Joseph Proust conducted experiments that demonstrated this law, from the late 1700's to the early 1800's. The Law of Definite Proportions is also sometimes called Proust's Law.
Examples of the Law of Definite Proportions
Let us take, for example, the compound, water. Whatever the source of water, its composition is that of two atoms of hydrogen and one atom of oxygen.
This figure shows that water, from any source, is always made up of two atoms of hydrogen and one atom of oxygen. If we calculate the molecular weight of water, we come up with 18 g/mol. In 1 mole of water, there are 2 grams of hydrogen and 16 grams of oxygen. By weight, we have a percentage of 11% hydrogen and 89% oxygen in one mole of water. This translates into a ratio of 1:8 of hydrogen to oxygen in water.
The Law of Definite Proportions illustrates that whatever the amount of water, whether it be 2 moles or 54 grams, the ratio of the amount of hydrogen to oxygen by weight will always be the same, just like the egg:sugar:butter ratio in chocolate cake should always be the same to maintain consistent taste.
Let's look at another compound made up of three atoms this time, such as ethyl alcohol, which consists of 2 atoms of Carbon, 6 atoms of Hydrogen and one atom of Oxygen.
According to the figure, we can calculate the percentage of each element in ethyl alcohol by weight, and we come up with 52% carbon, 13% hydrogen and 35% oxygen by weight. Based on the Law of Definite Proportions, the percentages of carbon, hydrogen and oxygen by weight in ethyl alcohol will always be the same, no matter what the amount.
Sample Problems
Let's look at some problems involving the Law of Definite Proportions in chemistry.
Problem 1
Sodium chloride, NaCl, contains 39% Na (sodium) and 61% Cl (chloride) by mass. If you have a 150-g sample of NaCl, how many grams of sodium and chloride are there in the sample?
Gallery Law Of Definite Composition
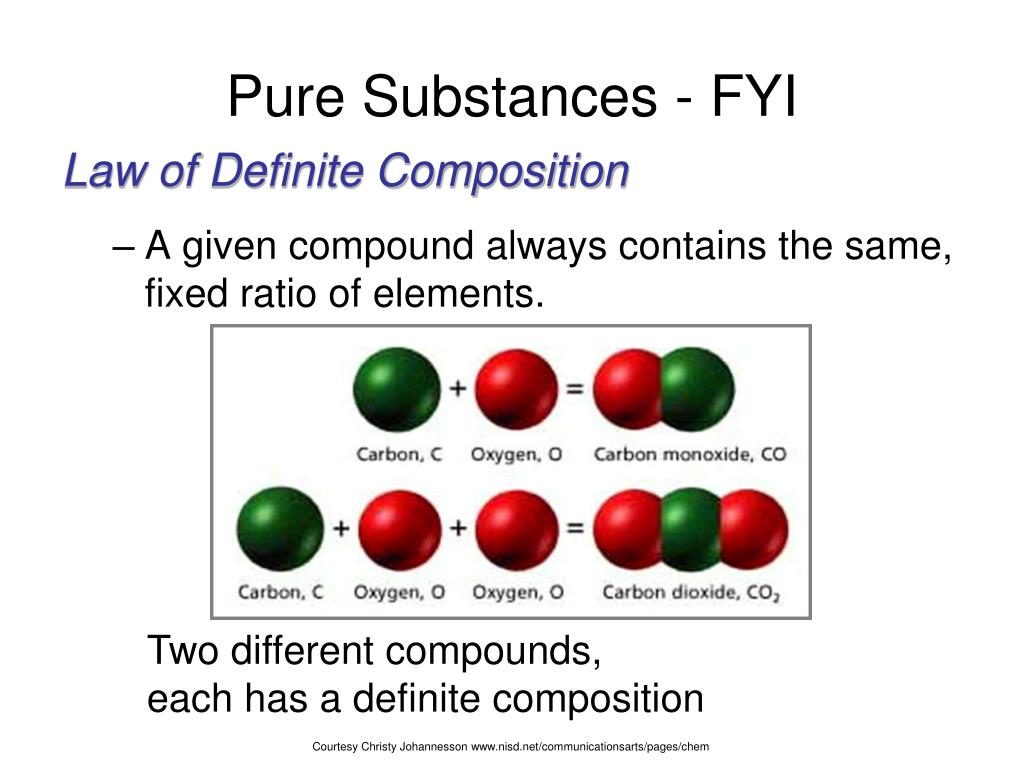 Ppt Classification Of Matter Powerpoint Presentation Free
Ppt Classification Of Matter Powerpoint Presentation Free
 Sat Chemistry Chemical Formulas Laws Of Definite
Sat Chemistry Chemical Formulas Laws Of Definite
 Chemistry 11 Law Of Definite Composition Multiple
Chemistry 11 Law Of Definite Composition Multiple
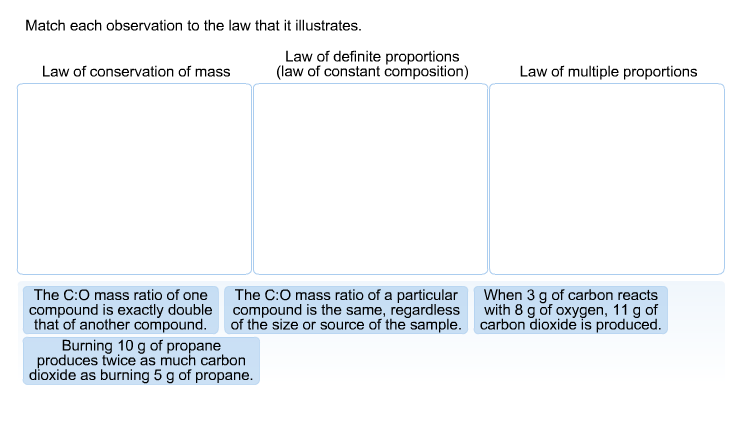 Solved Match Each Observation To The Law That It Illustra
Solved Match Each Observation To The Law That It Illustra
 What Is Law Of Conservation Of Mass Law Of Constant
What Is Law Of Conservation Of Mass Law Of Constant
 Scenes A I On The Next Page Depict Various Clutch Prep
Scenes A I On The Next Page Depict Various Clutch Prep
 Law Of Definite Composition And Law Of Multiple Proportions
Law Of Definite Composition And Law Of Multiple Proportions
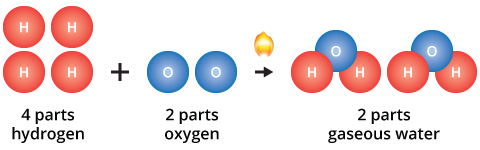 Early Ideas About Matter Chemistry Visionlearning
Early Ideas About Matter Chemistry Visionlearning

 Lab 1 The Law Of Definite Proportions Tri
Lab 1 The Law Of Definite Proportions Tri
 Law Of Definite Composition By Donna Bautista On Prezi
Law Of Definite Composition By Donna Bautista On Prezi
 Law Of Definite Proportions Law Of Multiple Proportions
Law Of Definite Proportions Law Of Multiple Proportions
 2 2 Scientific Laws Conservation Of Mass And Definite
2 2 Scientific Laws Conservation Of Mass And Definite
 The Law Of Definite Proportions Definition Examples
The Law Of Definite Proportions Definition Examples
 Law Of Definite Composition And Law Of Multiple Proportions
Law Of Definite Composition And Law Of Multiple Proportions
 Chemistry Timeline 1 1800 S Joseph Proust The Law Of
Chemistry Timeline 1 1800 S Joseph Proust The Law Of
 Ppt Chapter 4 Matter Energy Powerpoint Presentation
Ppt Chapter 4 Matter Energy Powerpoint Presentation
 Law Of Definite Proportions Or Proust S Law Chemistrygod
Law Of Definite Proportions Or Proust S Law Chemistrygod
Opinions On Law Of Definite Proportions
 Chapter One Chem Chem 107 Intro Chemistry Principles I
Chapter One Chem Chem 107 Intro Chemistry Principles I
:max_bytes(150000):strip_icc()/GettyImages-556421537-567836fd5f9b586a9e6ab717-5b993773c9e77c00504a4b6f.jpg) Law Of Definite Proportions Definition
Law Of Definite Proportions Definition
Ppt Law Of Definite Composition And Law Of Multiple
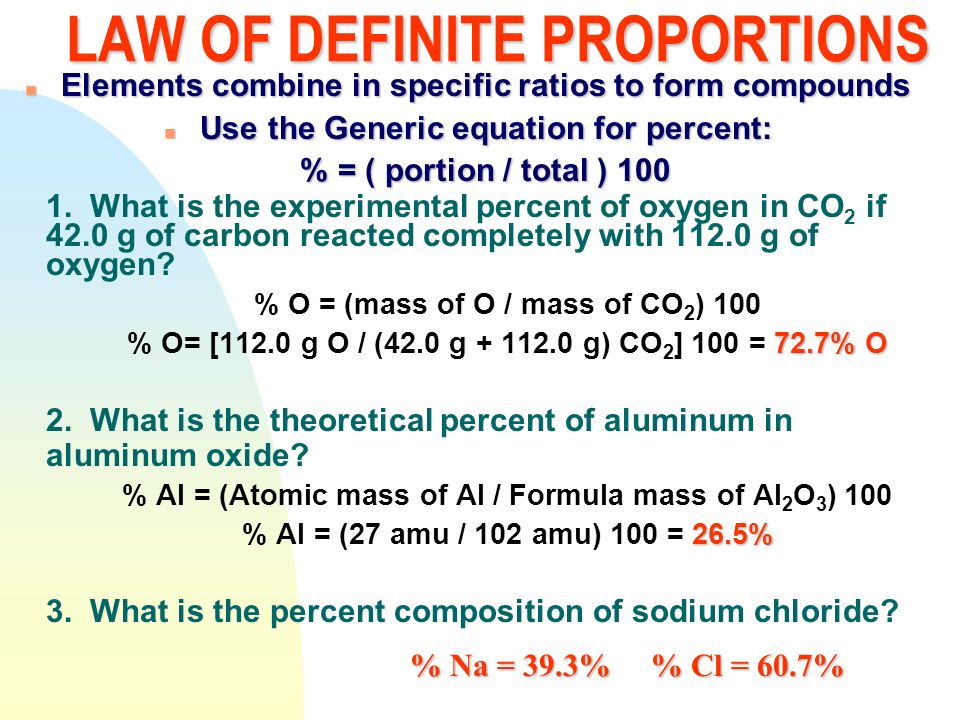 Law Of Definite Proportions Ppt Download
Law Of Definite Proportions Ppt Download
 Law Of Multiple Proportions And Law Of Definite Composition
Law Of Multiple Proportions And Law Of Definite Composition

Comments
Post a Comment